Description
March 16, 2021 08:30 ET |
Source: Brain Scientific Inc.
NEW YORK, March 16, 2021 (GLOBE NEWSWIRE) -- via InvestorWire -- Brain Scientific Inc. (OTCQB: BRSF), a neurology-focused medical device and software company, announces a collaboration with JelikaLite, a company developing Cognilum™, a non-invasive wearable photobiomodulation device that is being developed to increase the well-being of children living with autism, through a clinical trial titled “Transcranial Photobiomodulation for Reducing Autism Symptoms in Children.”
In the clinical trial, brain treatments for children diagnosed with autism would be delivered through Cognilum™, while Brain Scientific’s NeuroEEG, a wireless EEG device, will monitor brain activity. The clinical trial is designed to give researchers and clinicians an opportunity to potentially uncover new treatment options for reducing the symptoms children with autism experience.
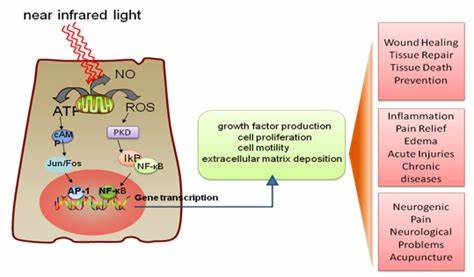
Based in New York, JelikaLite was launched by a group of medical researchers and practitioners specializing in psychology, neurology and phototherapy. JelikaLite’s Cognilum™ device is being developed to deliver treatment through transcranial photobiomodulation; the science uses infrared light, delivered through LEDs, to reduce brain inflammation and increase brain connectivity while the embedded sensors collect additional physical data from the patient. JelikaLite’s software platform would then provide an assessment of the child and capture ongoing data from the wearable device. Through the use of the platform’s learning modules and input of qualitative information, along with data analysis, JelikaLite believes the platform will provide progress reports and personalized recommendations for each child’s individual treatment, including efforts to decrease symptoms such as tantrum reduction, to increase eye contact and to improve speech.
“JelikaLite is developing a system called Cognilum that combines a home-based therapeutic wearable device, which applies near-infrared light therapy and records physical measurements with an intelligent AI platform. It provides personalization through data collection, analysis and reporting to improve a child's autism symptoms through gains in communication and daily living skills,” said Katya Sverdlov, CEO of JelikaLite.
Brain Scientific’s next-generation neurology products continue to serve researchers, clinicians and others working in brain diagnostics and treatment. The NeuroEEG device to be used in the clinical trial is a portable, wireless and compact device intended to acquire, record, transmit and display electrical brain activity for patients. NeuroEEG works in conjunction with NeuroCap, a hospital-grade disposable EEG headset featuring 19 active channels and 22 electrodes. The device can perform rapid EEG testing not only at medical centers but also in private homes, which supports the joint efforts in the work towards continuous monitoring of children with autism.
“We are delighted to begin this collaboration with JelikaLite. Our Neuro EEG solutions have a wide range of applications, and helping to monitor children with autism to improve their well-being would be an important step towards a wider adoption of affordable and accessible EEG systems like ours,” said Irina Nazarova, Director of Marketing at Brain Scientific, Inc.
The “Transcranial Photobiomodulation for Reducing Autism Symptoms in Children” clinical trial is set to begin in March 2021 and will feature 30 participants in two locations in New York City. Beyond the clinical trial, the two companies are planning to join efforts in building a wearable device for home use, featuring Brain Scientific’s technology from its disposable NeuroCap, to monitor brain activity of children with autism, including EEG measurements with near-infrared light (PBM) therapy.

